
Jungfrau.ch
Since the discovery of the ozone layer, countries have agreed and amended treaties to aid its recovery. The most notable of these is the Montreal protocol on substances that deplete the ozone layer, which is widely regarded as the most successful environmental agreement ever devised.
Ratified by every UN member state and first adopted in 1987, the Montreal protocol aimed to reduce the release of ozone-depleting substances into the atmosphere. The most well known of these are chlorofluorocarbons (CFCs).
Starting in 1989, the protocol phased out the global production of CFCs by 2010 and prohibited their use in equipment like refrigerators, air-conditioners and insulating foam. This gradual phase-out allowed countries with less established economies time to transition to alternatives and provided funding to help them comply with the protocol’s regulations.
Today, refrigerators and aerosol cans contain gases like propane which, although flammable, does not deplete ozone in Earth’s upper atmosphere when released. However, ozone-friendly alternatives to CFCs in some products, such as certain foams used to insulate fridges, buildings and air-conditioning units, took longer to find. Another set of gases, hydrochlorofluorocarbons (HCFCs), was used as a temporary replacement.

RichardJohnson/Shutterstock
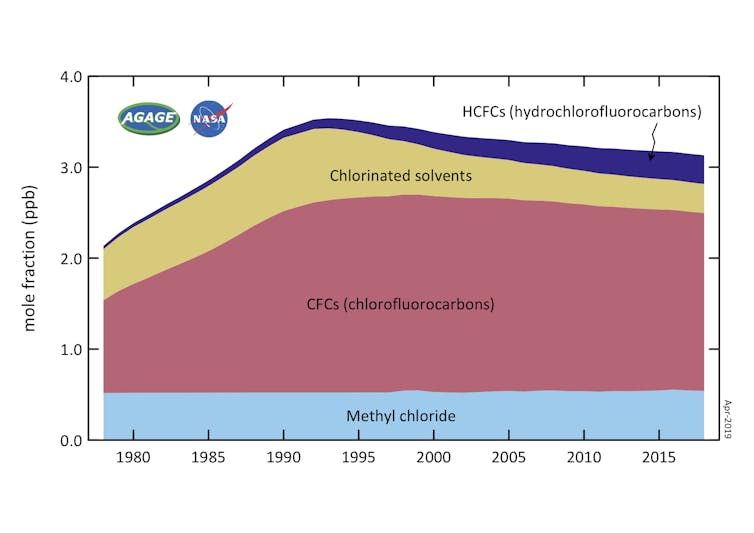
Unfortunately, HCFCs still destroy ozone. The good news is that levels of HCFCs in the atmosphere are now falling and indeed have been since 2021 according to research I led with colleagues. This marks a major milestone in the recovery of Earth’s ozone layer – and offers a rare success story in humanity’s efforts to tackle climate-warming gases too.
HCFCs v CFCs
HCFCs and CFCs have much in common. These similarities are what made the former suitable alternatives.
HCFCs contain chlorine, the chemical element in CFCs that causes these compounds to destroy the ozone layer. HCFCs deplete ozone to a much smaller extent than the CFCs they have replaced – you would have to release around ten times as much HCFC to have a comparable impact on the ozone layer.
But both CFCs and HCFCs are potent greenhouse gases. The most commonly used HCFC, HCFC-22, has a global warming potential of 1,910 times that of carbon dioxide, but only lasts for around 12 years in the atmosphere compared with several centuries for CO₂.
As non-ozone depleting alternatives to HCFCs became available it was decided that amendments to the Montreal protocol were needed to phase HCFCs out. These were agreed in Copenhagen and Beijing in 1992 and 1999 respectively.
This phase-out is still underway. A global target to end most production of HCFCs is set for 2030, with only very minor amounts allowed until 2040.
Turning the corner on a bumpy road
Our findings show that levels of HCFCs in the atmosphere have been falling since 2021 – the first decline since scientists started taking measurements in the late 1970s. This milestone shows the enormous success of the Montreal protocol in not only tackling the original problem of CFCs but also its lesser known and less destructive successor.
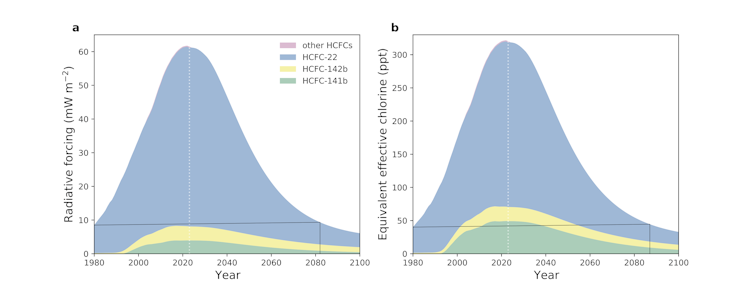
Western et al. (2024)/Nature
This is very good news for the ozone layer’s continuing recovery. The most recent scientific prediction, made in 2022, anticipated that HCFC levels would not start falling until 2026.
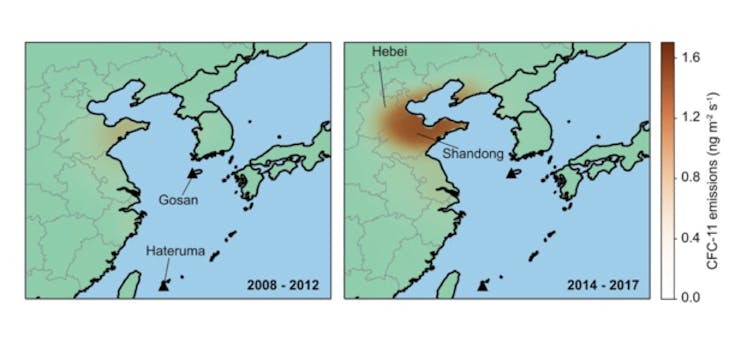
Despite HCFC levels in the atmosphere going in the right direction, not everything has been smooth sailing in the phase-out of ozone-depleting substances. In 2019 a team of scientists, including myself, provided evidence that CFC-11, a common constituent of foam insulation, was still being used in parts of China despite the global ban on production.
The United Nations Environment Programme also reported that HCFCs were illegally produced in 2020 contrary to the phase-down schedule.
In 2023, I and others showed that levels of five more CFCs were increasing in the atmosphere. Rather than illegal production, this increase was more likely the result of a different process: a loophole in the Montreal protocol which allowed CFCs to be produced if they are used to make other substances, such as plastics or non-ozone depleting alternatives to CFCs and HCFCs.
Some HCFCs at very low levels in the atmosphere have also been shown to be increasing or not falling fast enough, despite few or no known uses.
Most of the CFCs and HCFCs still increasing in the atmosphere are released in the production of fluoropolymers – perhaps best known for their application in non-stick frying pans – or hydrofluorocarbons (HFCs).
HFCs are the ozone-friendly alternative that was developed and commercialised in the early 1990s to replace HCFCs, but their role as a potent greenhouse gas means that they are subject to international climate emission reduction treaties such as the Paris agreement and the Kigali amendment to the Montreal protocol.
The next best alternative to climate-warming HFCs is a matter of ongoing discussion. In many applications, it was thought that HFCs would be replaced by hydrofluoroolefins (HFOs), but these have created their own environmental problems in the formation of trifluoroacetic acid which does not break down in the environment and, like other poly- and per-fluorinated substances (PFAS), may pose a risk to human health.

AndriiKoval/Shutterstock
HFOs are at least more energy-efficient refrigerants than older alternatives like propane, however.
Hope for the future
In discovering this fall in atmospheric levels of HCFCs, I feel like we may be turning the final corner in the global effort to repair the ozone layer. There is still a long way to go before it is back to its original state, but there are now good reasons to be optimistic.
Climate and optimism are two words rarely seen together. But we now know that a small group of potent greenhouse gases called HCFCs have been contributing less and less to climate change since 2021 – and look to set to continue this trend for the foreseeable future.
With policies already in place to phase down HFCs, there is hope that environmental agreements and international cooperation can work in combating climate change.
—————————–
This blog is written by Cabot Institute for the Environment member Dr Luke Western, Research Associate in Atmospheric Science, University of Bristol. This article is republished from The Conversation under a Creative Commons license. Read the original article.



















.jpg)







